
We Chan Hui Mui Tan Shu Qin Pui Pang Ping Penny Tok Wu Jiawen Hillgrovians Secondary 3 Weather Studies Project Just say it. The Scheldue
Observation of soil
Data Collected
Results of the Experiments
Do-you-know facts
Chemicals can be acidic, neutral or basic depending on how many hydrogen ions they have.
Hydrogen ions are really just protons, a small particle that has a positive charge.
Scientists can find out whether a chemical is acidic, neutral or basic by testing its pH.
They use a special paper, called litmus paper, to do the pH test.
MORE FACTS
Do you know what pH stands for?
What is an acid?
Testing for pH values
Credits Our 10 Steps to test soil
1. Wash apparatus with distilled water. 3. Add distilled water into beaker and stir. 4. Placed the folded filter paper on top of a new beaker.  5. Pour the mixture into the filtered paper.  6. Wait for mixture to be filtered.  7. Use a dropper to place various test solutions and paper into six different test tubes. 8. Use another dropper to place filtrate into the six test tubes. 


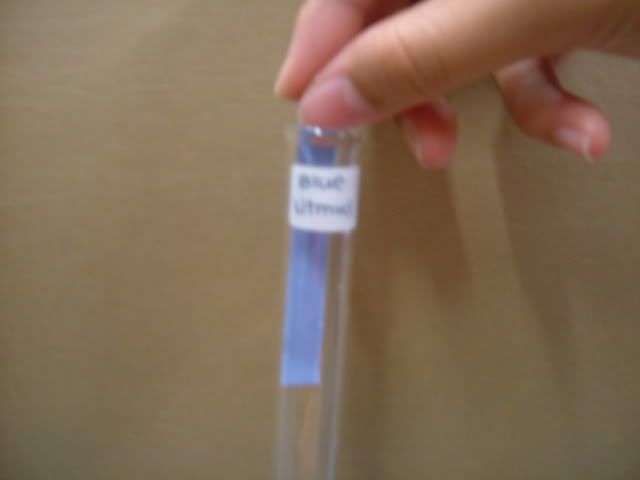
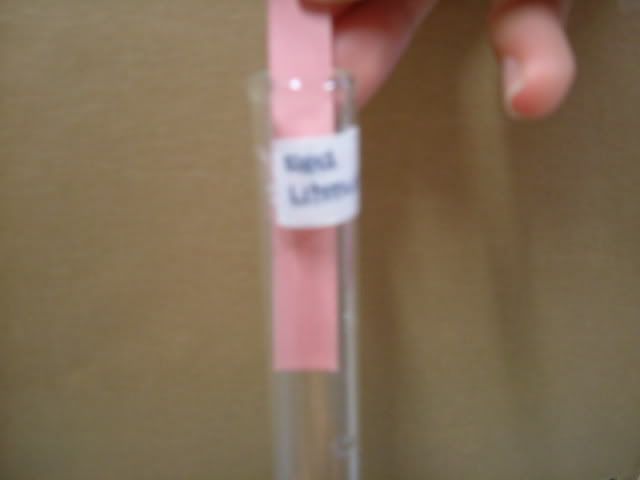
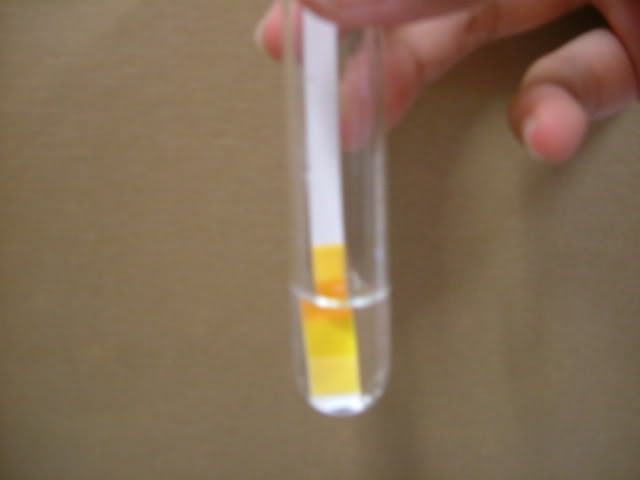
9. Use tweezer to take tested paper out of the test tube to place in zip lock bag 10. Take pictures of tested solution. Soil Pictures Locations
School For more information
Dirt on soil Archives November 2005 December 2005 January 2006 February 2006 March 2006 April 2006 May 2006 June 2006 July 2006 Contact us or alternatively, f4_chanhuimui@hotmail.com, tanshuqin@hotmail.com, classicjw@hotmail.com, sophisticated-p@hotmail.com. p.s please tag to inform us if you e-mailed. Count down! Visits
|
Monday, October 31, 2005 Today we went to MRL for a meeting.FOUR members out of SEVEN members did not turn up. There is sport leaders stuff and band stuff. This is our new blog. There is a new e-mail too. babyhilly@hotmail.com The password is WE-ALL-KNOW-IT So the conclusion of the meeting is... READ more references with the weather BRING materials such as -colouring materials -markers -pens -glue -scissors - cute cute stuff -stickers -chops NEXT MEETING will be on 081105 (tuesday) 9AM at MRL ALSO. Give me ALL your not-free dates. I will call you when i have a good hair day. You can e-mail it if you like but you must comment that you have e-mailed me. Or i will have no idea. Sms works too. carisse_@hellokitty.com | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
